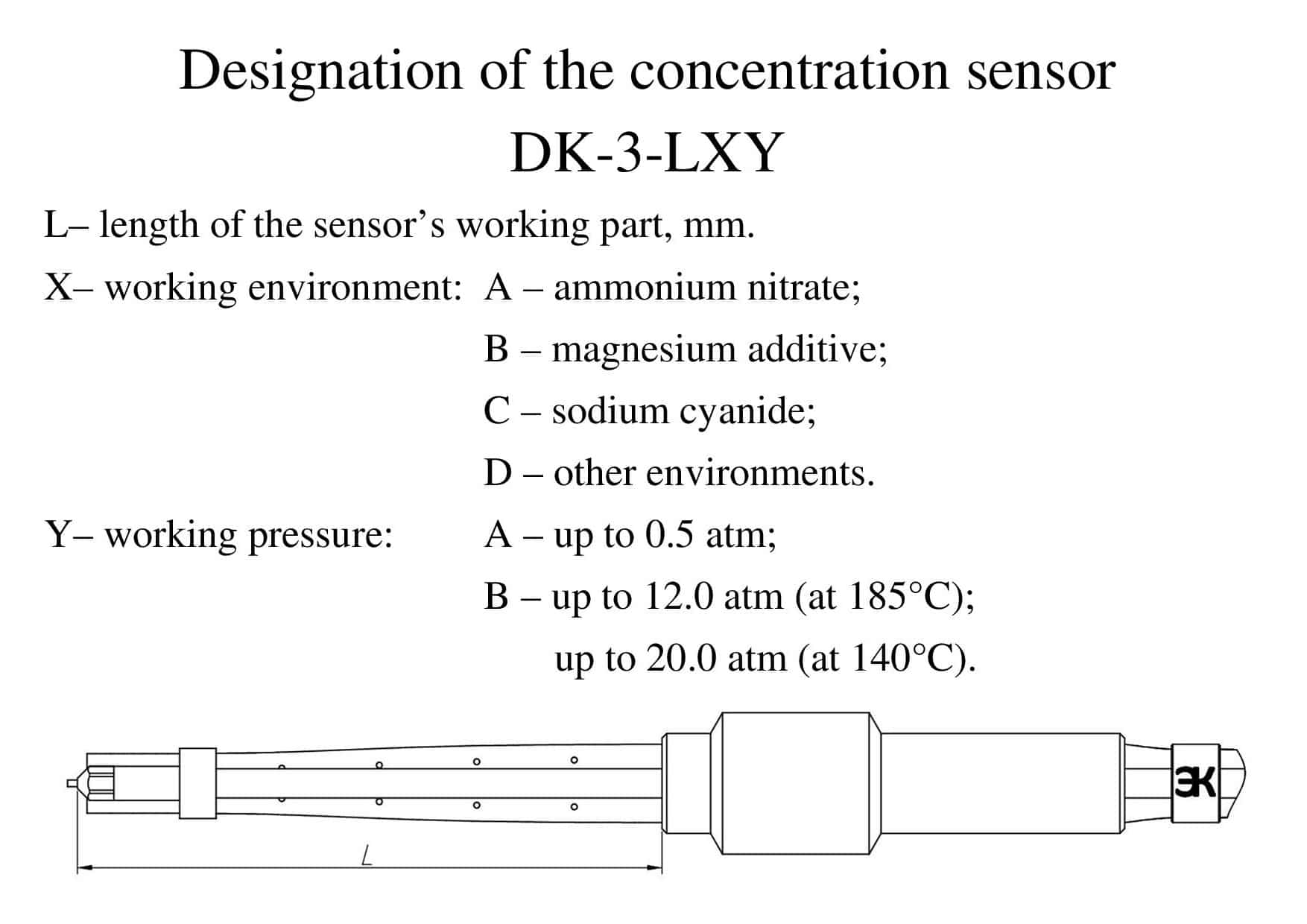
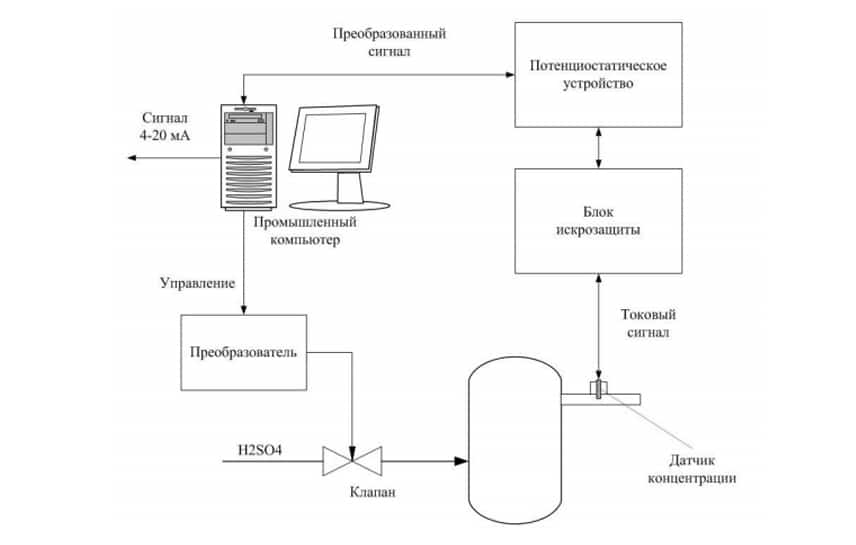
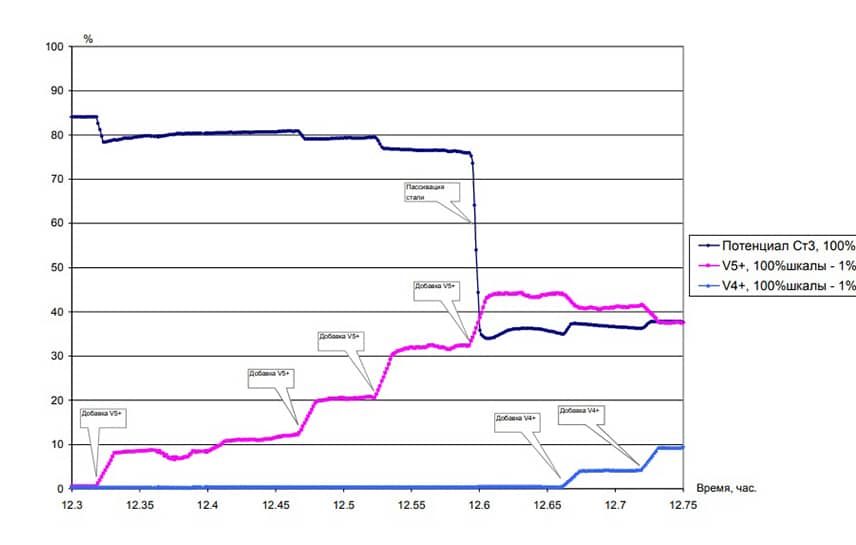
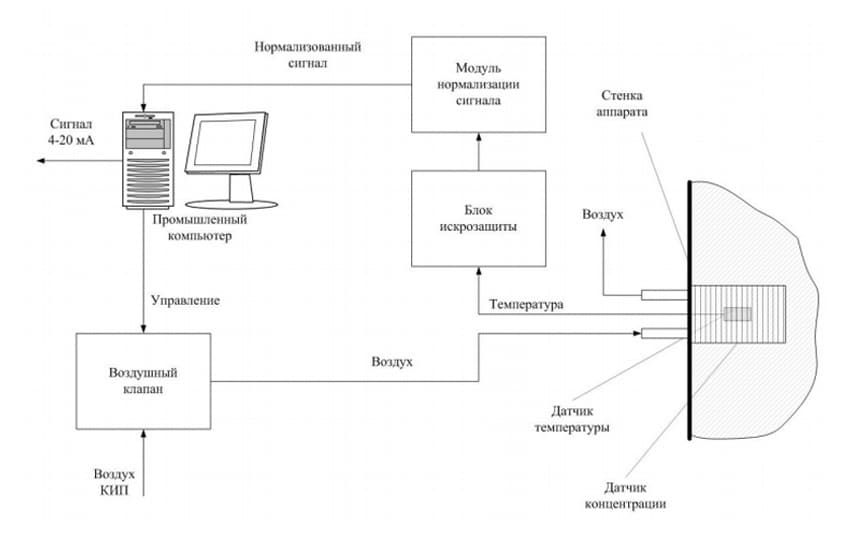
The system for monitoring of sulfuric acid concentration in the synthesis of hydroxylamine sulphate (for a single channel measurement of concentration) consists of:
- concentration sensor;;
- spark protection block;;
- potentiostatic device;
- industrial PC with I / O board.
The operation of the system is based on the relationship between redox currents on the polarized electrode in potentiodynamic mode and the of sulfuric acid concentration.
How the system works:
- At a signal from the computer potentiostatic device served potential difference across the concentration sensor; Current signal from the concentration sensor through the spark protection block is measured by potentiostatic device is converted into a voltage and fed to the ADC board computer;
- The computer calculates the magnitude of the signal on the concentration of sulfuric acid. On the basis of the monitoring system the system of regulation of the maintenance of sulfuric acid can be realized. The computer compares the measured value of the concentration of sulfuric acid with a given, calculates the control action, which comes with a DAC board to the valve through a conversion device.