Currently, the control (monitoring) of corrosion condition and control of corrosion speed of devices and pipelines in contact with aggressive environment, get the same value in order of importance as the control and regulation of the basic parameters of technological processes.
The active form of corrosion control provides for anticorrosive and technological measures that reduce the corrosion rate to acceptable values on the basis of received information. With the help of corrosion control systems can be efficiently solved the problems of control and corrosion protection of industrial equipment in sulfuric acid, phosphoric acid, hydrochloric acid, chloride, ammonia environments, as well as the circulating water, fertilizer solutions and solutions of gas purification from CO2. It was found that in aggregates of ammonia synthesis gas cleaning environment from CO2 are highly corrosive in relation to the carbon steel. To reduce the corrosion rate of the equipment in hot Benfield solutions is used as an inhibitor of vanadium pentoxide. However, the inhibitory (critical) concentration of V+5 – ions depends on several conditions – both process (temperature, CO2, etc.) and corrosion (steel surface condition, its potential). Currently, control of the content of the inhibitor and corrosion of equipment in the Benfield solution carry on the results of laboratory analyzes of vanadium ions and iron ions.
The main disadvantages of a laboratory method for the determination of vanadium ions and iron are the frequency analysis (once daily), as well as its complexity and subjectivity. The disadvantage of determining the corrosion rate by the corrosionelectrochemical methods in the laboratory is the complexity of modeling the conditions of the industrial apparatus (pressure up to 28 atm and temperatures above 100 °C, surface irrigation, the saturation of CO2, etc.).
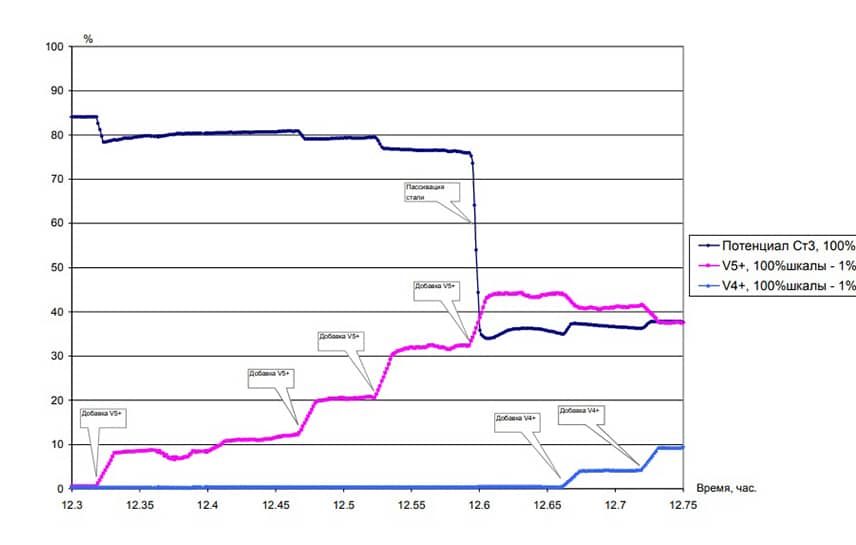
Research has shown that the most effective way to determine and maintain the passive state of equipment is control of corrosion state by the potential. There is a clear dependence between the rate of corrosion of carbon steel in Benfield solutions and the value of the corrosion potential. Therefore, the surface potential of the equipment reflects its state of corrosion most objectively. The sharp increase of the rate of corrosion of equipment is the result of a violation of the passive state.
At the same time to reduce the rate of corrosion of equipment to acceptable values you need an increased concentration of pentavalent vanadium, and to maintain a passive state concentration of V+5 – ions can be reduced in several times